How Cleanroom Molding Ensures Regulatory Compliance in Diagnostic Device Manufacturing
In-vitro diagnostic (IVD) devices, particularly those involving direct contact with patient specimens or reagents, demand manufacturing practices that meet the highest standards of cleanliness, traceability, and dimensional precision. Cleanroom injection molding is a critical component in the compliant production of such devices, ensuring both regulatory alignment and product integrity throughout the manufacturing lifecycle.
The Regulatory Imperative in Diagnostics
Diagnostic devices are subject to rigorous oversight under regulatory frameworks including the IVDR (EU 2017/746) in the European Union, 21 CFR Part 820 in the United States, and ISO 13485:2016 globally. These frameworks require a demonstrable control of bioburden, material compatibility, and manufacturing consistency, especially devices used in clinical diagnostics, molecular testing, or point-of-care applications.
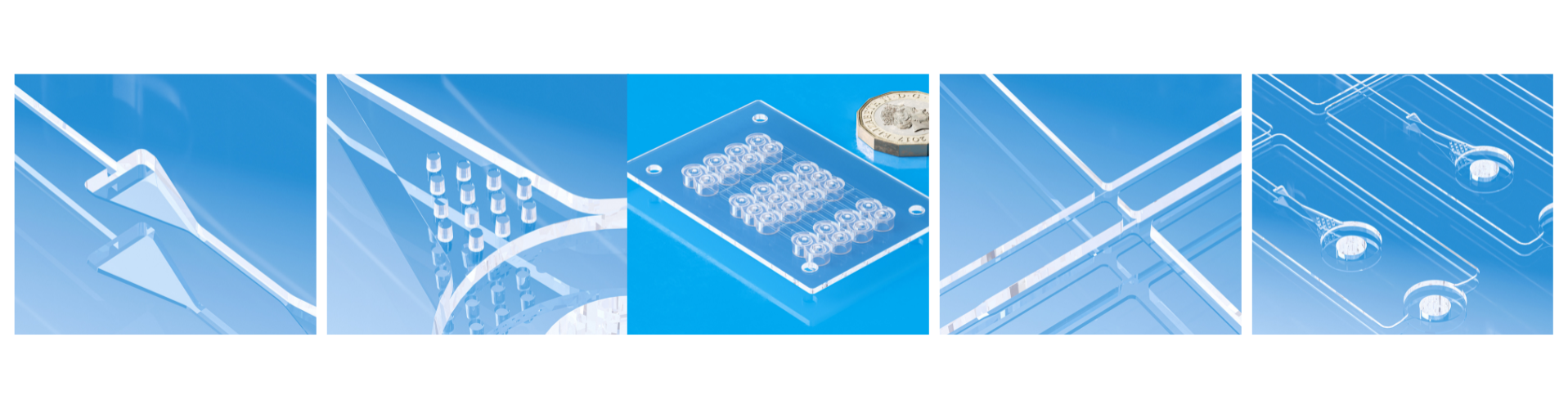
Components used in diagnostic cartridges, sample transfer devices, microfluidic chips, or reagent housing must follow production in conditions that minimize the risk of contamination or assay interference. Cleanroom injection molding provides the contamination control and process stability essential for compliance with these requirements.
Manufacturing diagnostics devices is a challenging sector in medical molding, require following strict global regulatory frameworks (Photo: Micro Systems)
Technical Overview: Cleanroom Injection Molding in a GMP Context
Cleanroom molding typically follows ISO Class 7 or Class 8 controlled environments, as defined by ISO 14644-1. The classification ensures control over airborne particulate matter ≥0.5 µm. This can impact both surface cleanliness and product performance.
In a GMP-compliant moulding environment, technical measures include:
- HEPA filtration ensuring ≥99.97% efficiency at 0.3 µm
- Environmental monitoring (EM) for viable and non-viable particulates
- Validated cleaning protocols to maintain aseptic conditions
- Controlled material flow and gowning procedures to minimize operator-derived contamination
Additionally, the entire production process is under a QMS aligned with ISO 13485, ensuring traceable documentation, batch record control, and design transfer traceability.

Cleanroom mold manufacture and injection molding facilities at Micro Systems
Key Technical Benefits for Diagnostic Applications
- Bioburden Control and Sterility Assurance
Diagnostic components such as cuvettes, pipette tips, or reaction chambers must be free from microbial contaminants. Molding in a cleanroom reduces initial bioburden, simplifying downstream sterilization or aseptic assembly processes. - Dimensional Stability for Precision Fluidics
High-precision tooling and closed-loop process control allow for dimensional tolerances within microns. This is critical in microfluidic diagnostics where fluid flow rates, capillary action, or optical alignment depend on precise geometries. - Polymer Compatibility and Leachables Management
Medical-grade polymers (e.g., COC, COP, PC, PP) must be processed under validated conditions to avoid degradation or release of leachables that could interfere with sensitive reagents or enzymatic reactions. - Particulate and Residue Control
The use of low-shear, medical-validated molding parameters ensures minimal generation of particulates. This is essential in optical diagnostic devices where clarity and absence of artefacts directly influence readout accuracy. - Traceability and Electronic Batch Record Integration
MES (Manufacturing Execution Systems) can integrate with Cleanroom molding systems, enabling real-time process monitoring, digital batch records, and data integrity in line with Annex 11 and 21 CFR Part 11 requirements. - Support for Sterile Barrier System Integration
Components destined for sterile barrier systems must meet ISO 11607 preconditions, including particulate cleanliness and structural integrity. Cleanroom molding facilitates this integration at source.

Micro Systems provides end-to-end support for diagnostic device development
Strategic Importance in Diagnostic Device Development
As diagnostic devices increasingly incorporate complex reagent-handling and multiplexing capabilities, the role of high-fidelity, contamination-free molded components has become indispensable. Working with a molding partner who offers end-to-end support, from material validation and DFM (Design for Manufacturability) to IQ/OQ/PQ validation protocols, ensures a robust regulatory position and accelerated time to market.
At Micro Systems, our Class 7 cleanroom facilities fully follow ISO 13485 standards. We specialize in high-precision micro molding for critical diagnostic applications, offering material traceability, statistical process control, and full regulatory documentation. We are the trusted partner to leading diagnostic brands across the UK and Europe.
Contact us today!