Advanced Trends in Medical Device Manufacturing
The medical device industry is experiencing significant growth, driven by technological advancements and an increasing demand for healthcare solutions. Molding technologies, particularly injection molding, play a pivotal role in this expansion. Here’s an in-depth look at the current trends and market statistics shaping the sector in 2025.
- Global Medical Device Market: Valued at approximately $678.88 billion in 2025, with a projected compound annual growth rate (CAGR) of 6% over the next decade (Arterex Medical)
- Medical Injection Molding Market: Estimated at $27.32 billion in 2025, growing at a CAGR of 7.2% (The Business Research Company)
- Medical Device Contract Manufacturing Market: Projected to reach $84.86 billion in 2025, with a CAGR of 10.86% (Grand View Research)
Overmolding in Medical Device Manufacturing
Overmolding involves the injection of a second material, typically a thermoplastic elastomer (TPE), over a rigid substrate to create a single, integrated component. This process enhances the functionality, ergonomics, and aesthetics of medical devices.
The global medical device overmoulding market was valued at approximately $4.5 billion in 2024 and is projected to reach $7 billion by 2030, growing at a CAGR of 7–8% (marketreportanalytics)
Applications in Medical Devices
- Handheld Diagnostic Devices: Such as blood glucose monitors and thermometers, where user comfort and ease of cleaning are paramount.
- Surgical Instruments: Providing ergonomic grips and reducing the risk of slippage during procedures.
- Drug Delivery Systems: Ensuring secure and comfortable interfaces for patients.
Advantages
- Enhanced Biocompatibility: Soft-touch surfaces improve patient comfort and compliance.
- Reduced Part Count: Combines multiple components into a single part, simplifying assembly.
- Improved Durability: Provides resistance to wear and tear, extending the device’s lifespan.
- Compliance with Regulatory Standards: Facilitates adherence to hygiene and safety regulations.
Challenges
- Material Compatibility: Ensuring proper adhesion between dissimilar materials.
- Design Complexity: Requires careful consideration of material properties and processing conditions.
- Cost Implications: Higher initial tooling costs and material expenses.

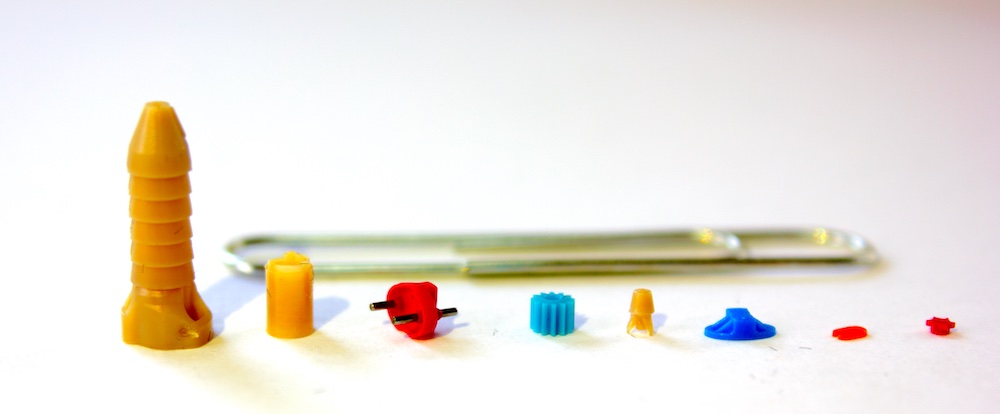
Micro Molding in Medical Device Manufacturing
Micro molding is a precision injection molding process that produces components with extremely small dimensions, often in the micrometre range. This technique is essential for manufacturing miniaturized medical devices.
The medical micro injection molding market was valued at $374.7 million in 2025 and is projected to reach $1.66 billion by 2033, growing at a CAGR of 12.2%. (Archive Market Research)
Micro molding has become the standard for next-generation medical devices, driven by the rise of minimally invasive and wearable technologies.
Applications in Medical Devices
- Microfluidic Devices: For diagnostics and laboratory testing.
- Implantable Sensors: Monitoring patient conditions in real-time.
- Drug Delivery Systems: Ensuring precise dosing and delivery.
- Surgical Instruments: Providing high precision for delicate procedures.
Advantages
- High Precision: Achieves tight tolerances, often below ±10 µm.
- Miniaturization: Facilitates the development of compact and lightweight devices.
- Cost Efficiency: Reduces material waste and energy consumption.
- Scalability: Enables high-volume production of complex parts.
Challenges
- Tooling Complexity: Requires advanced mould designs and materials.
- Process Sensitivity: Minor variations can lead to significant defects.
- Material Limitations: Not all materials are suitable for micro-moulding processes.
High-Performance Polymers and Material Engineering
The global medical polymers market is projected to grow from $44.70 billion in 2025 to $66.29 billion by 2030, at a CAGR of 8.2% (MarketsandMarkets).
High-performance polymers, including PEEK, PPSU, and bioresorbable materials, are increasingly utilized in medical devices due to their superior mechanical properties and biocompatibility.
Applications
- Implantable Devices: Materials like PEEK are used in spinal and dental implants for their strength and radiolucency.
- Diagnostic Equipment: PPSU is employed in diagnostic imaging components due to its high thermal stability.
- Drug Delivery Systems: Bioresorbable polymers are utilized in controlled drug release applications.
Challenges
- Material Compatibility: Ensuring consistent performance across different sterilization methods.
- Cost: High-performance polymers are often more expensive, impacting overall device cost.
Process Optimization and Automation
Technological Advancements
- The integration of AI and machine learning in injection molding processes is enhancing precision and reducing errors.
- Robotic arms and vision systems are increasingly used to expedite repetitive tasks, boosting safety and production speed.
Market Impact
Automation is shifting from optional to essential in cleanroom molding cells, with high-precision robotics and in-line process monitoring becoming standard
Benefits
- Increased Efficiency: Automation reduces cycle times and increases throughput.
- Enhanced Quality Control: Real-time monitoring ensures consistent product quality.
- Cost Reduction: Minimizes human error and reduces labour costs.
Sustainability and Lifecycle Considerations
Environmental Initiatives
Medical device manufacturers are adopting sustainable practices across the product lifecycle, from raw material sourcing to end-of-life management.
Market Trends
The demand for eco-friendly materials and processes is growing, driven by regulatory pressures and consumer preferences.
Challenges
- Material Sourcing: Identifying sustainable materials that meet performance standards.
- Recycling: Developing effective recycling methods for medical devices.
Regulatory and Quality Engineering
Compliance Standards
Manufacturers must adhere to stringent regulations such as ISO 13485 and ISO 10993 to ensure device safety and efficacy.
Quality Assurance
Implementing robust quality management systems is essential for maintaining product integrity and meeting regulatory requirements.
Challenges
- Documentation: Maintaining comprehensive records for regulatory audits.
- Testing: Ensuring thorough testing to meet biocompatibility and performance standards.

Micro Systems specializes in precision molding solutions for the medical device industry, combining expertise in micro molding, overmolding, and high-performance polymers. Their advanced manufacturing facilities integrate automation, real-time process monitoring, and strict quality management systems to ensure components meet the most stringent regulatory and biocompatibility standards. By leveraging cutting-edge technologies and sustainable practices, Micro Systems enables medical device manufacturers to reduce production costs, accelerate time-to-market, and deliver innovative, high-precision devices that improve patient outcomes.
Contact us today!