Regulatory Standards for Sustainable Medical Device Manufacturing (ISO 14001, EU MDR, FDA guidance)
Sustainable medical device manufacturing is now a critical regulatory priority. Global healthcare supply chains must meet stringent safety and performance requirements while demonstrating measurable reductions in energy use, emissions, and material waste. This technical article examines three principal frameworks, ISO 14001 environmental management systems, the EU Medical Device Regulation (EU MDR 2017/745), and emerging FDA guidance, and details their impact on injection-molded medical devices, biocompatible polymer selection, and circular-economy manufacturing strategies.

Sustainability as a Compliance Imperative
Healthcare contributes nearly 5 % of global greenhouse gas emissions, making low-carbon manufacturing of medical devices a regulatory and market necessity (WHO, 2023). Manufacturers of single-use plastics, including high-volume injection molded medical components, face increasing scrutiny over carbon footprints, waste streams, and end-of-life impacts.
Key regulatory drivers include:
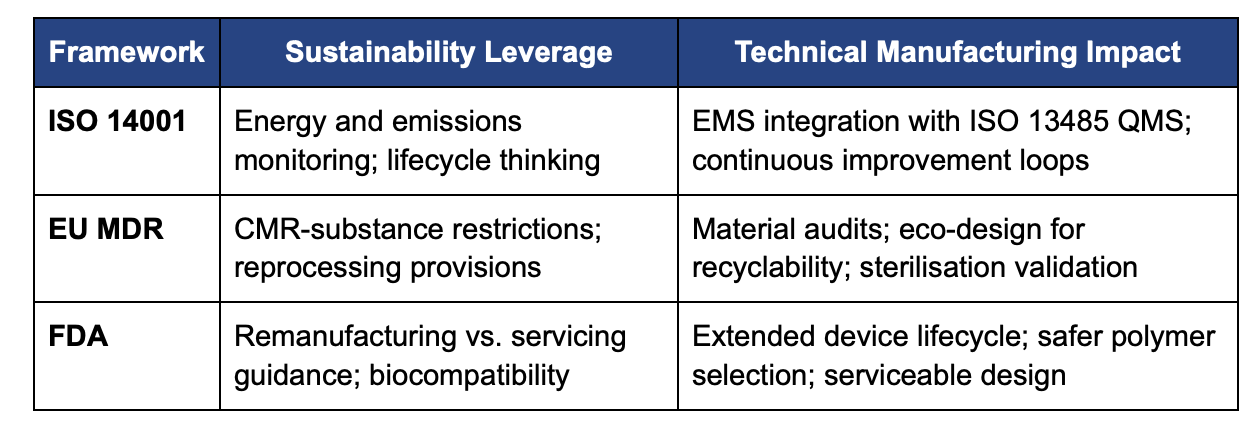
- ISO 14001:2015 for Environmental Management Systems (EMS) [ISO, 2015].
- EU MDR (Regulation (EU) 2017/745) with embedded environmental and chemical-safety requirements [European Parliament, 2017].
- FDA remanufacturing and biocompatibility guidances, which extend device lifecycle expectations [FDA, 2024a; FDA, 2023].
ISO 14001: Framework for Environmental Management
ISO 14001 establishes a risk-based, life-cycle approach to environmental performance. Core technical requirements include:
- Identification of environmental aspects, energy consumption of injection molding machines, volatile organic compound (VOC) emissions, resin scrap, and water usage.
- Compliance evaluation against national and international legislation.
- Quantifiable objectives and key performance indicators (KPIs) for carbon reduction, waste minimization, and supply-chain sustainability.
Practical applications in medical injection molding:
- Hot-runner tooling to minimize cold-runner waste and reduce cycle energy.
- Closed-loop polymer recycling, feeding sprues and runners back into medical-grade production when biocompatibility standards permit.
- Renewable-energy integration, with some plants achieving >30 % CO₂ reduction post-certification (Freudenberg Medical, 2024).

EU MDR: Regulatory Mandates with Environmental Impact
General Safety & Performance Requirements (Annex I)
Manufacturers must justify any use of carcinogenic, mutagenic or reprotoxic (CMR) substances above 0.1 % w/w and design for safe disposal [European Parliament, 2017].
Reprocessing of Single-Use Devices (Article 17)
Validated reprocessing and sterilization can convert single-use devices to reusable status, provided the reprocessor assumes full manufacturer responsibility and maintains ISO 13485-aligned quality systems [European Commission, 2024].
Interaction with Other EU Law
Compliance intersects with REACH, RoHS, and WEEE directives, requiring evidence of hazardous-substance control and post-market environmental reporting [ECHA, 2023].
Technical Implications for Injection Molding
- Material selection: biocompatible, low-additive polymers with verifiable REACH compliance.
- Design for disassembly to enable recycling of multi-material devices.
- Lifecycle documentation integrated into the technical file for Notified Body review.
FDA Guidance: Lifecycle Extension and Material Safety
Although the FDA does not yet impose a dedicated sustainability rule, several guidances support circular-economy healthcare:
- Remanufacturing Guidance (2024) clarifies when refurbishment or repair constitutes “remanufacturing,” encouraging manufacturers to supply maintenance protocols that prolong device life [FDA, 2024a].
- ISO 10993-1 Biological Evaluation Guidance (2023) demands rigorous chemical-characterisation and toxicological risk assessments, incentivising the use of safer, lower-impact polymers [FDA, 2023].
- Labelling requirements increasingly call for maintenance and service instructions, reducing premature device disposal [FDA, 2024a].
Implementation Strategies for Eco-Design Medical Devices
- Advanced Material Audits: Evaluate every polymer and additive against EU MDR CMR thresholds and FDA biocompatibility guidance [European Parliament, 2017; FDA, 2023].
- High-Precision Process Control: Deploy IoT sensors and AI predictive maintenance to cut cycle energy by up to 20 % in injection molding lines (Industry Data, 2024).
- Life-Cycle Assessment (LCA): Quantify cradle-to-grave impacts to meet ISO 14001 continual-improvement requirements [ISO, 2015].
- Supplier Sustainability Agreements: Require upstream ISO 14001 certification and verifiable ESG reporting (Freudenberg Medical, 2024).

Challenges and Opportunities
- Data Integration: Combining ISO 14001 EMS with ISO 13485 Quality Management Systems requires harmonized metrics.
- Cost vs. ROI: High-performance biopolymers may carry a 10–15 % price premium but lower total cost of ownership through reduced waste and regulatory risk.
- Upcoming EU Ecodesign Regulation: Anticipated requirements for durability, repairability and recyclability will tighten the regulatory landscape (European Commission, 2024).
The convergence of ISO 14001, EU MDR, and FDA guidance creates a binding framework for sustainable medical device manufacturing. For injection-molded devices, early adoption of eco-design principles, low-carbon energy sources, biocompatible polymers, closed-loop recycling, reduces environmental footprint while safeguarding regulatory compliance and market competitiveness.
References
ECHA. (2023). Legislation profile: EU Medical Devices Regulation. European Chemicals Agency.
European Commission. (2024). Reprocessing of single-use medical devices.
European Parliament. (2017). Regulation (EU) 2017/745 on medical devices. Official Journal of the European Union.
FDA. (2023). Use of ISO 10993-1, Biological Evaluation of Medical Devices—Part 1. U.S. Food & Drug Administration.
FDA. (2024a). Remanufacturing of Medical Devices: Final Guidance. U.S. Food & Drug Administration.
Freudenberg Medical. (2024). Global ISO 14001 Certification Press Release.
ISO. (2015). ISO 14001:2015 Environmental Management Systems—Requirements with Guidance for Use.International Organization for Standardization.
WHO. (2023). Health-care climate footprint report. World Health Organization.