Design for Manufacturability (DFM) in Medical Injection Molding
Medical injection molding demands not only mechanical precision and repeatability, but also compliance with biocompatibility, sterilization protocols, traceability, and regulatory frameworks. Applying Design for Manufacturability (DFM) from the earliest design stages can reduce cost, minimize defects, shorten time to market, and ensure safety in medical device applications.
What Is DFM in Medical Injection Molding?
Design for Manufacturability (DFM) involves designing parts in such a way that they are producible efficiently, with high yields, minimal rework, and full adherence to required specifications. In medical injection molding, this also means meeting regulatory requirements (e.g. ISO 13485, ISO 10993), ensuring material safety, sterilisation compatibility, and maintaining dimensional stability under use and sterilization cycles [4].
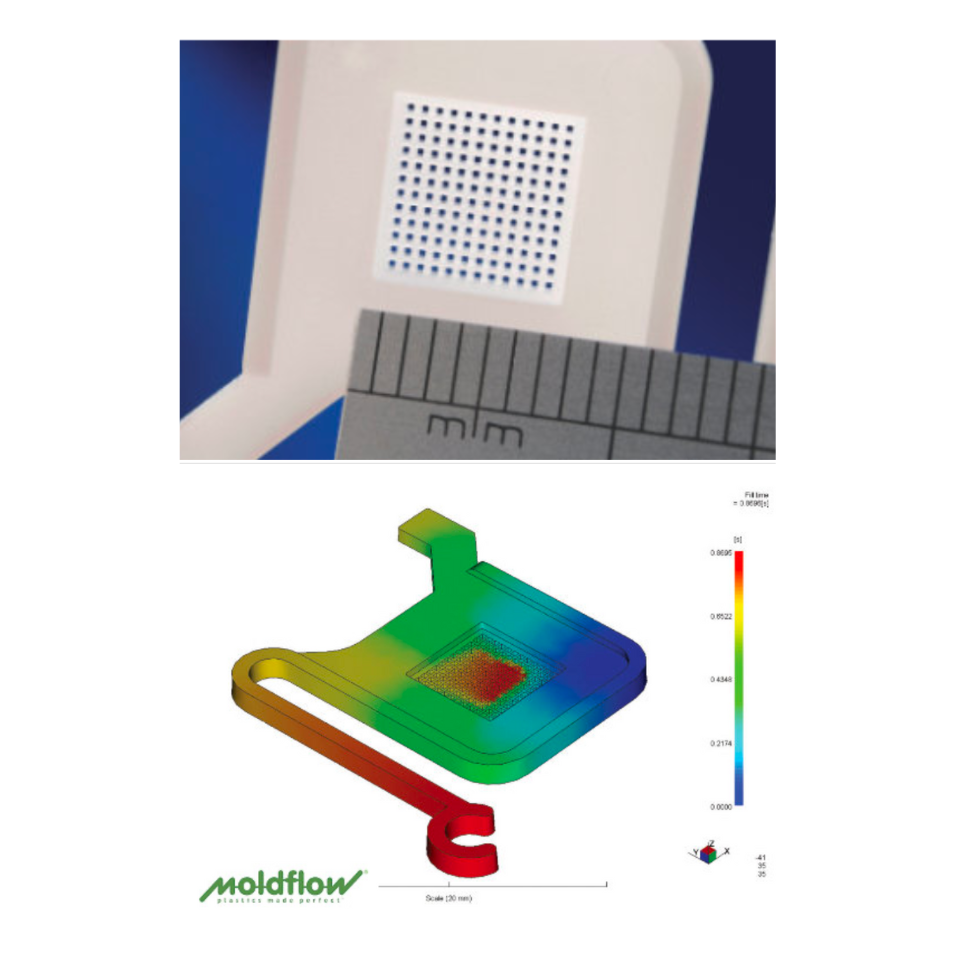
Key DFM Principles for Medical Injection Molded Parts
Uniform Wall Thickness
Maintaining uniform or near-uniform wall thickness is fundamental. Variations in wall thickness can introduce warpage, sink marks, voids, and uneven cooling. As a rule of thumb, adjacent walls should be no less than 40-60% of each other’s thickness [1] [6]. Excessively thick walls can also increase cycle times and residual stress in the finished part [5].
Ribs, Bosses
- Ribs: Use ribs to provide reinforcement rather than thickening walls. Rib thickness should typically be no more than ~60% of the nominal wall thickness to avoid sink marks and warping [17] [9]. Rib height should often not exceed ~2.5-3× nominal wall thickness [15] [11].
- Bosses: Boss bases thick relative to adjacent walls can cause voids or sink marks; keep boss base thickness ≤ 60% of adjacent wall where cosmetic appearance or surface integrity matter [9] [17].
- Gussets: Gussets strengthen corners or junctions between walls and ribs without adding unnecessary bulk. They should be tapered and kept thin, generally no more than the adjoining wall thickness, to prevent sink marks and to ensure uniform cooling and stress distribution. Adequate draft (at least 1°) and filleted roots help with ejection and reduce stress concentrations [1] [15].
Draft Angles
Surfaces that are nearly vertical in the mold should have adequate draft angles to permit ejection without damage. The amount of draft needed depends on material, texture, depth of cavity etc. [1] [15].
Surface Finish & Corners
Sharp internal corners concentrate stress and inhibit flow; use radii and fillets to smooth corners. Internal radii of about 0.25–0.5× the wall thickness are often used where possible [3] [11]. Textures or gloss surfaces require more careful draft and finishing to avoid drag lines / blemishes [13].
Cooling, Venting & Material Flow
Balanced filling of the mold helps avoid weld lines, air traps. Proper venting is required to allow air escape, especially in deep cavities or narrow features. Cooling channels should be designed to maintain uniform temperature to avoid hot spots [4] [5].
Regulatory, Material & Validation Constraints
Regulatory Compliance: ISO 13485 is the standard for quality management systems in medical device manufacturing; companies must document process controls, traceability, risk management etc. [0] [4]. Other relevant standards include ISO 10993 (biocompatibility), cleanroom standards such as ISO 14644-1, and MDR (EU/UK) / FDA regulations depending on region [4].
Material Selection: Materials must be biocompatible, capable of withstanding sterilization (autoclave, radiation, ethylene oxide, etc.), and should have stable mechanical, chemical and optical properties under expected use conditions [4].
Validation & Quality Assurance: Processes such as IQ/OQ/PQ (Installation, Operational, Performance Qualification) are needed to validate that the moulding process reliably produces conforming parts. Statistical process control (SPC), first article inspection (FAI), documentation of lot numbers of materials, process settings, and environmental conditions are required under ISO 13485 [0] [10].
Typical Design Workflow with DFM in Medical Injection Molding
- Requirements Definition: Function, sterilization method, biocompatibility, regulatory class, lifecycle.
- Preliminary Design: CAD models incorporating uniform walls, materials, draft, simplified geometry. Engage with mold tool suppliers to assess tool feasibility.
- Simulation & Revision: Use mold-flow, thermal, cooling simulations to predict defects (warpage, sink marks, filling etc.), iterate design.
- Tooling & Prototype: Design mold tool with surface finish, venting, gates, runners; prototype or bridge tooling may be used for validation.
- Validation / Qualification: IQ/OQ/PQ, material / sterilization trials, dimensional verification, quality control implementation.
- Production & Monitoring: Batch traceability, process control, cleanrooms, audits, CAPA (Corrective and Preventive Actions).
Common Challenges & Mitigation Strategies
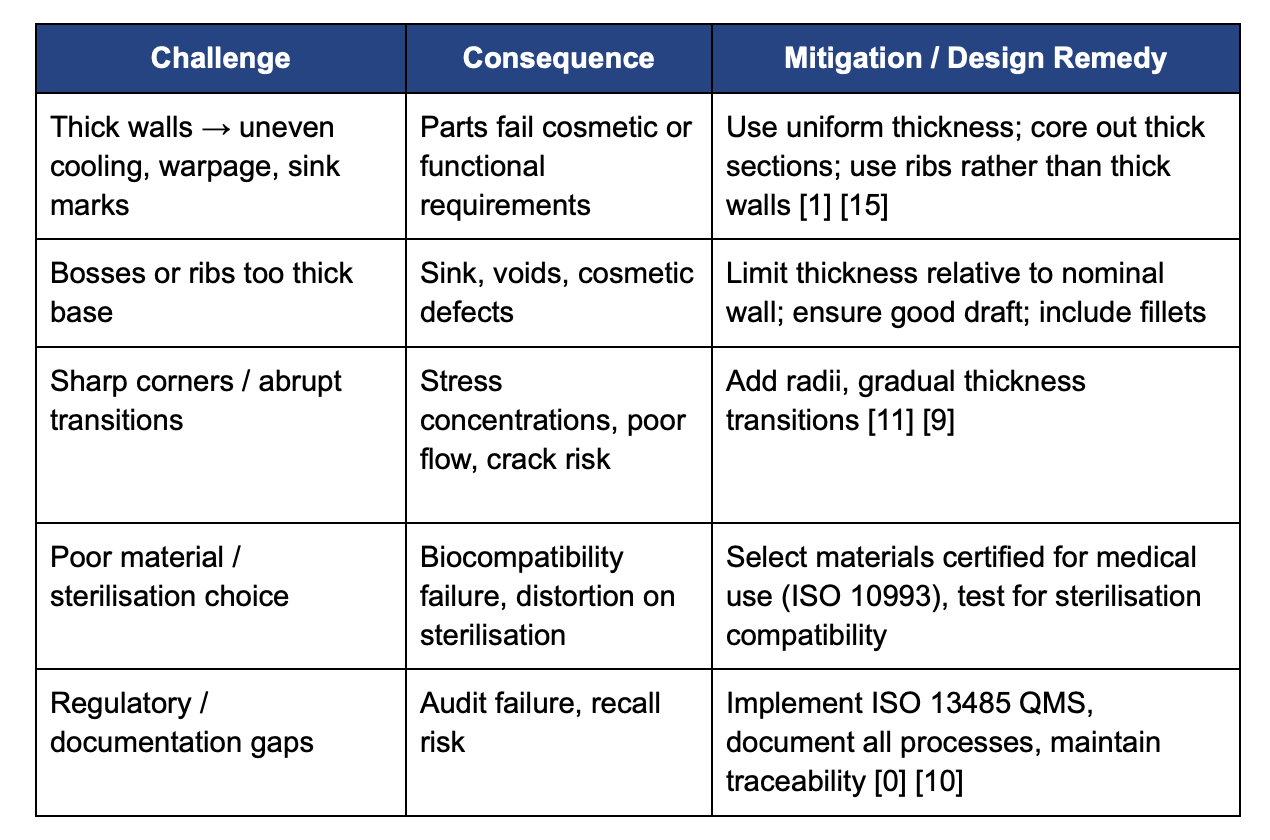
Design for Manufacturability in medical injection molding is indispensable. By integrating safe and feasible geometry (uniform walls, ribs, bosses, draft), anticipating regulatory and sterilization demands, and validating every stage, engineers and medical-device manufacturers can ensure quality, safety, cost-efficiency, and speed to market. Early cross-functional involvement (design, materials, tooling, regulatory) is essential to avoid costly redesigns and ensure compliance.
Why partnering with Micro Systems for DFM in Medical Injection Molding
Micro Systems (UK) offers ISO 13485-certified ultra-precision mold design, tooling, and cleanroom injection molding tailored to medical devices. Their in-house team supports every stage, from DFM consultation and material selection to mold manufacture, validation (IQ/OQ/PQ), and full-scale production, ensuring regulatory compliance, tight tolerances, and optimised tooling for complex or micro-scale components.
Contact us today!
References
[1] Protolabs. Design Essentials for Injection Moulding (2024). Available at: https://www.protolabs.com/en-gb/resources/design-tips/design-essentials-for-injection-moulding/
[3] Fictiv. Injection Molding Design Guide (2023). Available at: https://www.fictiv.com/articles/injection-molding-design-guide
[4] Aprios. Guide to Medical Injection Molding: Considerations and Best Practices (2025). Available at: https://www.aprios.com/insights/guide-to-medical-injection-molding-considerations-and-best-practices
[5] Rapiddirect. Injection Molding Design Guidelines (2024). Available at: https://www.rapiddirect.com/blog/injection-molding-design-guidelines/
[6] Protolabs. DFM Analysis for Injection Moulding (2023). Available at: https://www.protolabs.com/en-gb/resources/design-tips/dfm-analysis-injection-moulding/
[9] Texas Injection Moulding. Designing Ribs and Bosses in Injection Moulded Parts (2023). Available at: https://www.texasinjectionmolding.com/design-tips-ribs-bosses
[10] RPWorld. 4 Critical Considerations for Safe and Compliant Medical Injection Molding (2024). Available at: https://www.rpworld.com/blog/medical-injection-molding-considerations
[11] Dassault Systèmes SolidWorks. Injection Molding Design Guidelines (2024). Available at: https://blogs.solidworks.com/tech/injection-molding-design-guidelines
[13] 3D Systems. Basics of Injection Molding Design (2024). Available at: https://www.3dsystems.com/quickparts/knowledge-base/basics-injection-molding-design
[15] HLH Rapid. Injection Moulding Design Tips and Best Practices (2024). Available at: https://www.hlhrapid.com/injection-moulding-design-tips
[17] Xometry. Injection Moulding Design Guide (2024). Available at: https://xometry.eu/en/resources/injection-molding-design-guide/
[0] Medical Micro Molding / Micro Systems. Company Overview and Services (2025). Available at: https://www.medicalmoulds.com/