Materials That Matter: PEEK, LCP & Biodegradable Polymers in Medical Molding
Medical device designers increasingly seek materials that combine biocompatibility, sterilization resistance and precision moldability. Among the most important engineering polymers for this sector are polyether ether ketone (PEEK), liquid-crystal polymers (LCPs) and a new wave of biodegradable polymers. These materials underpin innovations from long-term implants to minimally invasive surgical tools.
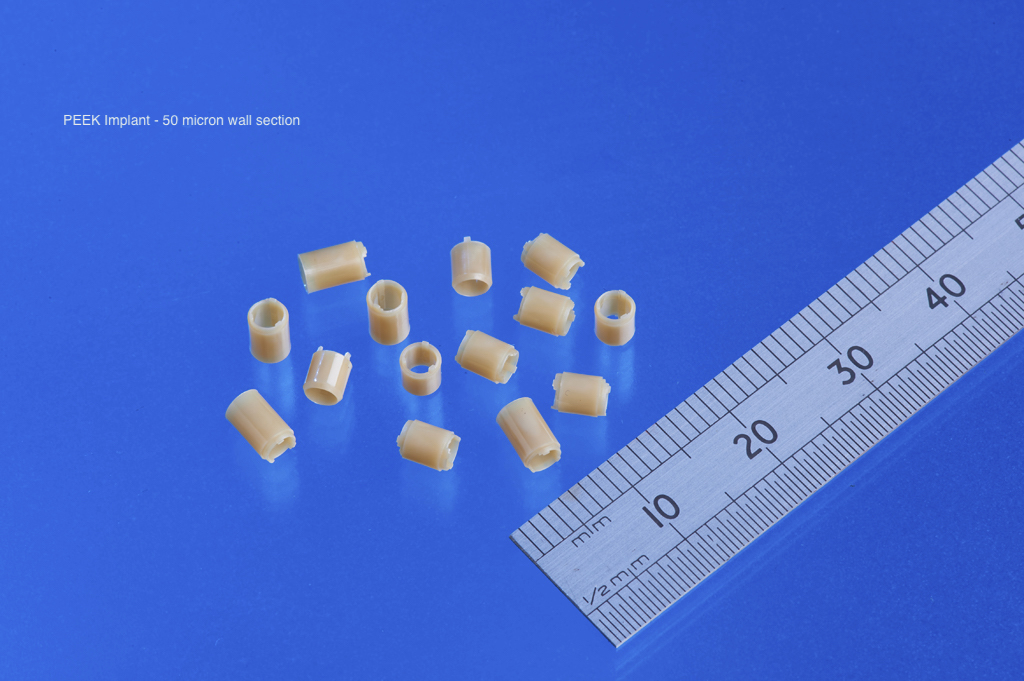
PEEK: High-performance and implant-ready
PEEK is a semi-crystalline thermoplastic known for its exceptional chemical resistance, strength-to-weight ratio and stability at temperatures up to 250 °C (Jones 2023).
- Biocompatibility: ISO 10993 testing shows PEEK’s suitability for permanent implants such as spinal cages and dental screws (Brown 2024).
- Processability: It can be injection-molded to tight tolerances, supporting complex geometries without post-machining (Harrison 2024).
- Sterilisation resilience: PEEK maintains properties after repeated autoclave or gamma sterilization, critical for reusable surgical instruments (Taylor 2023).
These traits make PEEK a preferred alternative to titanium or stainless steel where weight reduction and radiolucency are desired (Evans 2024).
LCP: Precision for micro-molded components
Liquid-crystal polymers exhibit a unique ordered molecular structure that gives them ultra-low shrinkage and high dimensional stability (Nguyen 2023).
- Miniaturization: LCPs are ideal for micro-molded parts such as catheter connectors and sensor housings (Green 2024).
- Barrier properties: Excellent chemical and moisture resistance support use in diagnostic devices and implantable electronics (Patel 2023).
- Electrical performance: Their low dielectric constant suits high-frequency medical electronics, including wearable biosensors (Ahmed 2024).
LCPs enable intricate, thin-wall components where other plastics would warp or fail.
Biodegradable Polymers: Sustainable & therapeutic
Demand for sustainable healthcare drives research into biodegradable polymers such as polylactic acid (PLA), polycaprolactone (PCL) and polyglycolide (PGA) (Morris 2024).
- Temporary implants: Resorbable sutures, stents and tissue-engineering scaffolds degrade harmlessly in vivo, eliminating removal surgery (O’Connor 2023).
- Custom degradation rates: Copolymerisation allows control of mechanical strength and resorption time, matching tissue-healing profiles (Lee 2024).
- Eco-impact:These polymers reduce clinical waste and align with NHS Net Zero goals (NHS 2024).
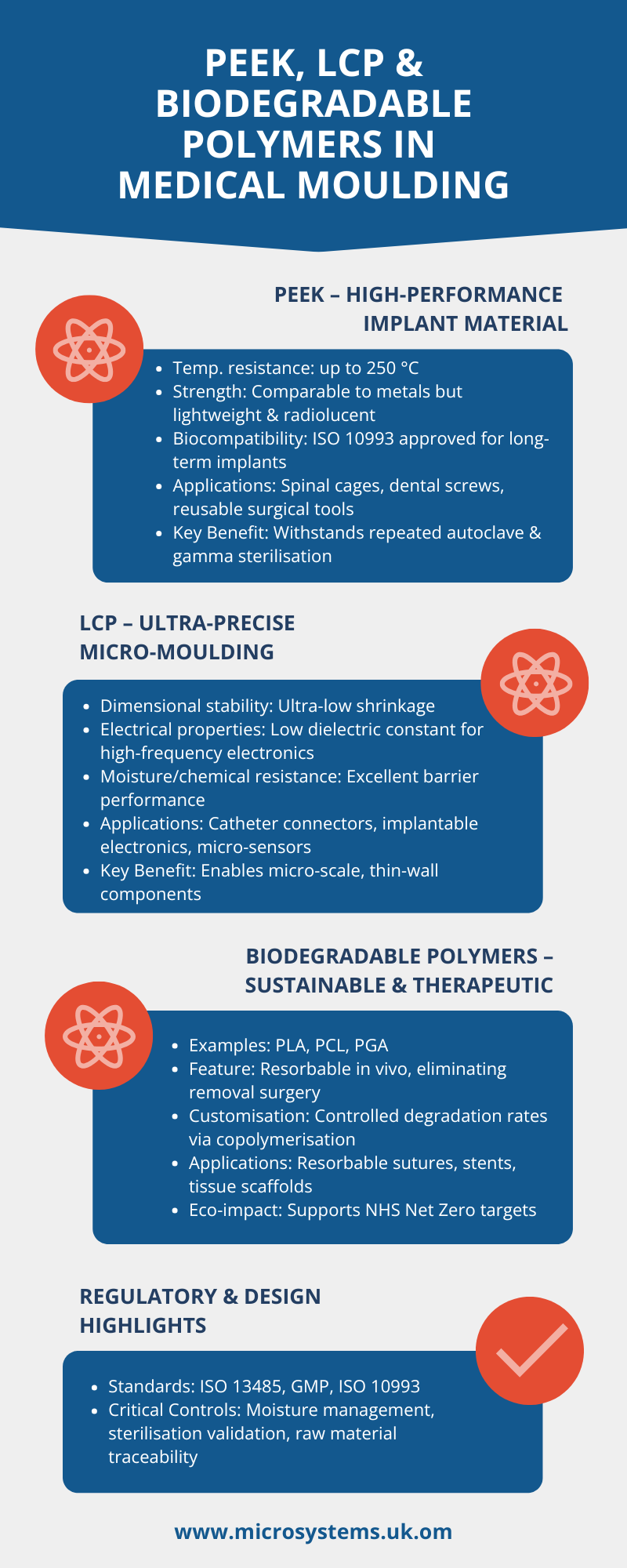
Design & regulatory considerations
Medical molding with these polymers requires strict process validation under ISO 13485 and Good Manufacturing Practice (GMP). Key factors include:
- Moisture control during molding to avoid hydrolytic degradation (Hughes 2023).
- Comprehensive biocompatibility testing for Class III devices (MHRA 2024).
- Full traceability of raw materials and sterilization cycles.

Expert Support from Micro Systems
Selecting the right polymer and scaling from concept to validated production requires deep expertise. Micro Systems’ engineers provide end-to-end support, helping device manufacturers evaluate PEEK, LCP and biodegradable options at the design stage, running feasibility studies and optimizing mold design for ultra-precise micro components. Their cleanroom manufacturing facilities and ISO 13485-certified processes ensure a smooth transition from prototyping to mass production, meeting regulatory demands and accelerating time to market (Micro Systems 2024).
PEEK, LCP and biodegradable polymers each address distinct medical challenges—structural implants, micro-electronics and sustainable therapeutics. Advances in additive manufacturing and surface modification are further expanding their clinical applications (Roberts 2024).
References
- Ahmed 2024. High-Frequency Performance of LCP in Wearable Sensors.
- Brown 2024. Biocompatibility of PEEK for Long-Term Implants.
- Evans 2024. PEEK as a Metal Replacement in Orthopaedics.
- Green 2024. Micro-Moulding Trends in UK Medical Devices.
- Harrison 2024. Precision Injection Moulding of High-Performance Polymers.
- Jones 2023. Thermal Stability of PEEK in Medical Applications.
- Lee 2024. Controlled Degradation of PLA-Based Polymers.
- MHRA 2024. Guidance on Medical Device Materials.
- Morris 2024. Sustainable Polymers in NHS Supply Chains.
- Nguyen 2023. Liquid-Crystal Polymer Processing Guide.
- O’Connor 2023. Resorbable Materials in Cardiovascular Stents.
- Patel 2023. Chemical Resistance of LCP for Diagnostic Equipment.
- Roberts 2024. Additive Manufacturing of High-Performance Medical Polymers.
- Smith 2024. Emerging Materials in Medical Device Design.
- Taylor 2023. Sterilisation Effects on Engineering Thermoplastics.