Pulmonary drug delivery
What is Pulmonary drug delivery?
Pulmonary drug delivery is a fast growing drug delivery system technology that targets the lower airways and lungs directly by inhaling drugs via the mouth. Among the many pulmonary medication delivery techniques are nebulizers, dry powder inhalers, and metered dose inhalers. When compared to alternative medication administration methods, pulmonary drug delivery offers several benefits, including quick drug absorption and non-invasiveness.
In Pulmonary drug delivery, when medicine is breathed through the mouth, it can directly target the lower airways and lungs. Treatment for respiratory diseases can be accessed directly through the lung.
The market for pulmonary drug delivery systems is growing fast, mainly due to the increasing preference of pulmonary route of drug delivery, new technology and an increase in the number of pulmonary diseases. According to a recent report, the pulmonary drug delivery market was valued at USD 55.26 billion in 2022 and is expected to reach around USD 92.4 billion by 2032, with a compound annual growth rate (CAGR) of 6.6% between 2023 and 2030.
What are the benefits of Pulmonary drug delivery?
Pulmonary drug delivery is a preferred route for lung disease treatment thanks to its capability to deliver extremely small drug particle size. Regarding the inhalation method, inhaled particle characteristics are one of the most important factors. Drug deposition via this route is also more effective because of the alveolar epithelium’s thickness (between 0.5 and 1.0 μm) and the large absorptive surface area (between 70 and 100 m2) (Nongkhlaw et al., 2020). Smaller particles that are inhaled and have a diameter of less than 5 μm may settle in the trachea and major bronchi, but bigger particles are stuck in the mouth and pharynx and can be absorbed gradually, especially if swallowed. It is possible for particles with a diameter of 2 to 5 μm to enter the tiny airways (Rogliani et al., 2017)(Sorino et al., 2020)(Virchow et al., 2008). Other factors that affect the deposition of aerosol particles in the lúng include aerosol physicochemical properties (shape, density, hygroscopicity) and patient conditions (the general health of , breathing pattern, etc.)
Besides, Pulmonary drug delivery is also the most recommended method of administering medication for lung conditions, including asthma and chronic obstructive pulmonary disease (COPD) because of the lungs’ huge surface area and weak physical barrier to absorption. Drugs delivered directly to the lungs may have a quicker start of action, be more effective at lower dosages, and have less systemic side effects (Sorino et al., 2020). For the treatment of respiratory disorders, Pulmonary drug delivery offers direct access to the site of illness without the inefficiencies and undesirable side effects of systemic medication administration.
In addition, as an alternative to intravenous and subcutaneous injection, pulmonary medication administration is noninvasive. Since inhaled drugs have been around for a while, they are commonly acknowledged as the best mode of administration for treating obstructive pulmonary disorders including asthma.
What are the different types of Pulmonary drug delivery?
Nowadays, there are a wide variety of pulmonary drug delivery techniques available, thanks to revolutionary particle engineering techniques that have transformed drug formulations. The market for pulmonary drug delivery has been influenced by the rising need for new medications to address a range of illnesses, which has increased focus on creating novel delivery strategies.
Dry Powder Inhalers (DPI)
Dry powder inhalers (DPIs) use tiny, tiny medicinal particles that can fit through even the tiniest airways to deliver medication in the form of a dry powder. DPIs have a powder formulation that is usually an ordered blend of bigger carrier lactose particles needed to optimise powder flow characteristics and micronized medication (less than 5 μm in diameter). An alternate formulation style that is employed in certain DPIs comprises cospheronised drug plus lactose or micronised drug particles alone that are loosely aggregated into tiny spherules.
The benefits of DPIs include ease of use (as users do not have to time their breath), no propellants (so less risk of environmental consequences) and resistance to contamination like bacteria, viruses and fungi. However, due to its powder characteristics, DPIs are more sensitive to moisture, so powder may cling inside the device as a result of water or saliva interfering with the medicine dissemination mechanism. In case of emergency, users might not be able to breathe deeply enough to suck out the powder if their airways have closed. It has also been recorded that older people might struggle to breathe quickly or deeply while using DPIs.
Metered Dose Inhalers (MDI)
Metered dose inhalers, or MDIs, are devices that provide medication via inhalation into the lungs, commonly with particle sizes of less than 5 μm. The Metered Dose Inhaler is a pressurised medication canister that administers medication as a spray or mist straight into the lungs of the user. It is frequently used to address respiratory issues such as asthma, chronic obstructive pulmonary disease (COPD), and other breathing disorders. When utilised appropriately, just 20% of the medication will enter the airway. When the remaining portion reaches the oropharynx, it is swallowed.
Every inhaler is made up of a mouthpiece and a little drug canister. When in use, users apply pressure on the pressurised canister, and breathe in slowly for 3 to 5 seconds, then hold the breath for 10 seconds to allow medicine to go dêply into their lungs.
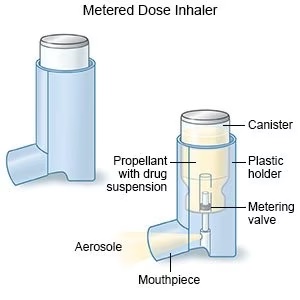 A typical Metered Dose Inhaler (Photo: Drugs.com)
A typical Metered Dose Inhaler (Photo: Drugs.com)
Nebulizers
Using a mouthpiece that is attached, a nebulzser creates a mist that users inhale to take in liquid medication. Additionally, nebulizer medication absorbs into the lungs more quickly, allowing it to enter the circulation faster and offer relief as soon as feasible. While both inhalers and nebulizers deliver medicine into the lungs, nebulizer is an electric device that sprays a thin liquid mist of medication through a mask or mouthpiece, and an inhaler is a portable device that is often used to inhale medicine in the form of a spray together with a spacer. Nebulizers are used for a number of different medications, including bronchodilators, hypertonic saline solutions and antibiotics for treating and preventing infections.
Compared to other inhalers, nebulizers are often simpler to use when it comes to administering medication. A little kid may find it difficult to stay motionless while inhaling the entire dose of medication through a nebulizer, which can take up to ten minutes to administer. In addition, inhalers are usually more compact and easier to travel with than nebulizers, which can be cumbersome to bring around.
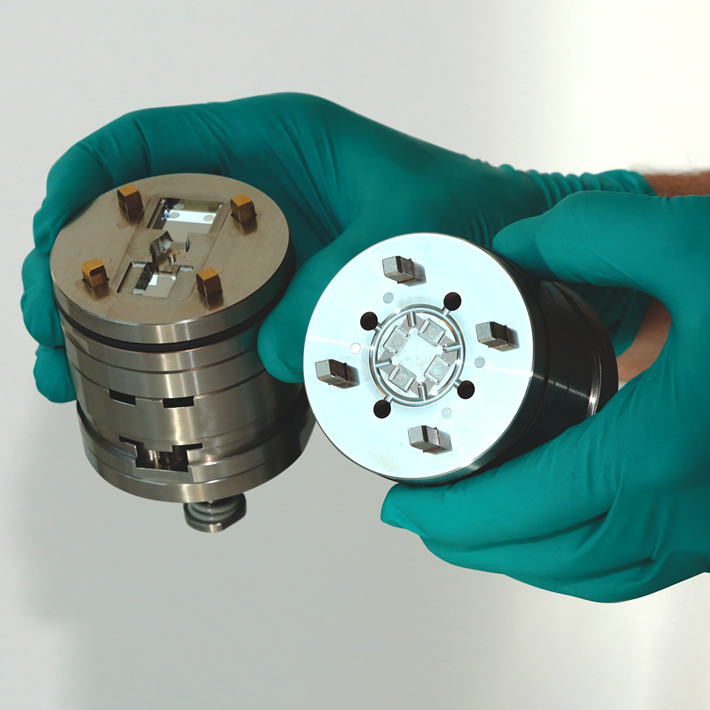
Ultra-precision molds for inhaler manufacture
In order to effectively produce on a mass production scale for delicate drug delivery systems like inhalers, designers and medical devices producers need to opt for advanced engineering techniques like ultra-precision mold manufacture and injection molding. The ultra precision of the injection molding procedure can affect the fit and functionality of the device, the accuracy of the dosage, hence the general performance of the device. It is hence crucial for pulmonary drug delivery system developers to work closely with manufacturers with significant experiences in ultra-precision injection molding, ideally someone with in-house mould design and manufacture capabilities, such that the whole project could be completed with efficiency and cost-effectiveness.
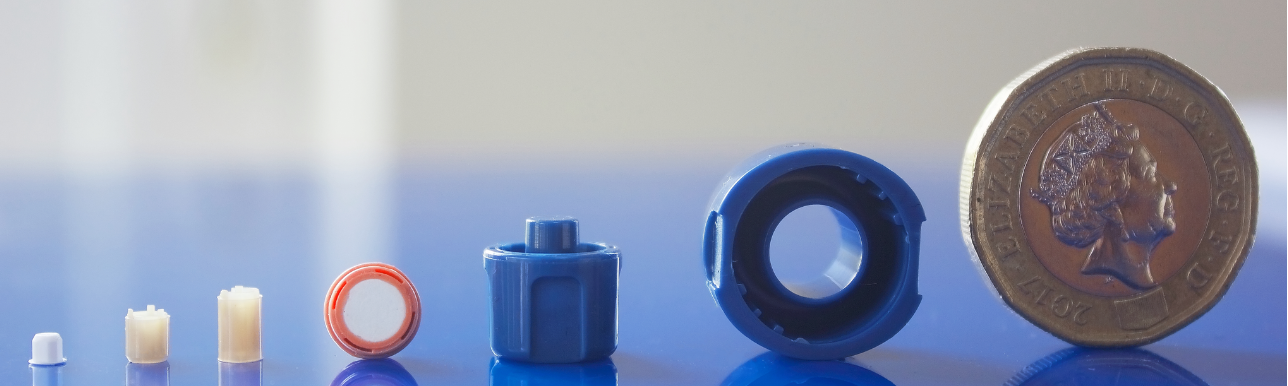
Photo: Micro System’s has extensive experiences in ultra precision mold design, mould manufacture and injection molding (Micro Systems)
Using our extensive knowledge and expertise in micro mold manufacture and precision injection molding, Micro Systems produces the best medication delivery devices for our medical device clients. WIth our ISO Class 7 and 8 cleanrooms, ISO 9001 and ISO 13485 accreditations, and the latest technology in micro engineering and injection moulding, we can cater all your pulmonary drug delivery requirements, from prototyping to mass production.
Contact us today to discuss your Pulmonary drug delivery projects!
Reference:
Nongkhlaw, R. et al. (2020) ‘Biologics: Delivery options and Formulation Strategies’, Drug Delivery Aspects, pp. 115–155. doi:10.1016/b978-0-12-821222-6.00006-3.
Sorino, C. et al. (2020a) ‘Inhalation therapy devices for the treatment of obstructive lung diseases: The history of inhalers towards the ideal inhaler’, European Journal of Internal Medicine, 75, pp. 15–18. doi:10.1016/j.ejim.2020.02.023.
Rogliani, P. et al. (2017) ‘Optimizing drug delivery in COPD: The role of Inhaler Devices’, Respiratory Medicine, 124, pp. 6–14. doi:10.1016/j.rmed.2017.01.006.
Virchow, J.C. et al. (2008) ‘Importance of inhaler devices in the management of airway disease’, Respiratory Medicine, 102(1), pp. 10–19. doi:10.1016/j.rmed.2007.07.031.