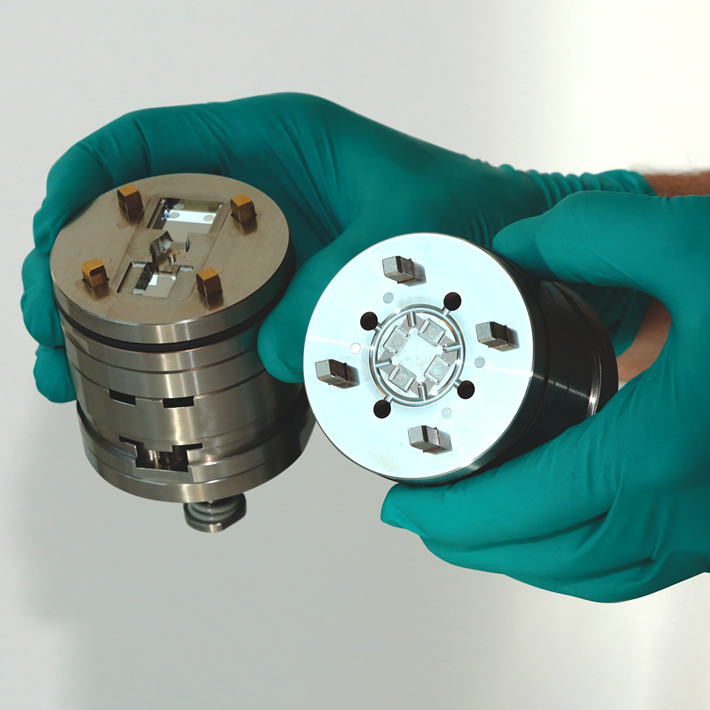
The commercialization of Microfluidic devices

(Photo: Micro Systems)
What is Microfluidics?
Microfluidics could be defined as the science and engineering of systems in which the behavior of fluid is different from conventional flow theory mainly due to the small length scale of the system (Nguyen et al., 2019). Microfluidics combines the profound knowledge within the field of the physics of fluids, at the same time, the engineering and expertise of designing and manufacturing devices with extremely small features.
This technology’s market worth has been slowly rising to a billion dollar value as it is being used by many sectors and academic disciplines. According to the most recent study by Emergen Research, the size of the worldwide microfluidics market reached USD20.14 billion in 2021 and is anticipated to experience a revenue CAGR of 16.1% during the projected period. By 2030, the microfluidics market size will reach USD 77.28 billion, with the driving factor being the higher demand from the healthcare industry. The microfluidics market is anticipated to have considerable global expansion during the forecast period as a result of the continued development of novel microfluidics technologies such 3-D printed microfluidics, paper-based microfluidics, and droplet-based microfluidics. Additionally, the incorporation of artificial intelligence (Al) and machine learning (ML) in microfluidic devices is expanding as a result of the potential of these technologies for real-time analysis and decision-making in a variety of applications.
Microfluidic devices mass manufacturing methods are increasingly important for the transition from laboratory prototype to potential commercialisation and clinical testing in the real world. The manufacture of microfluidic devices involves a number of phases due to the complexity of the microfluidic component itself, including design, prototyping, optimization, manufacturing micro tools, precise replication, surface treatment, and integration of the whole device together. Due to its lower cost, flexibility and adaptability, one of the main materials for the mass manufacture of the microfluidic devices is plastic, which has been successfully employed in the industry.
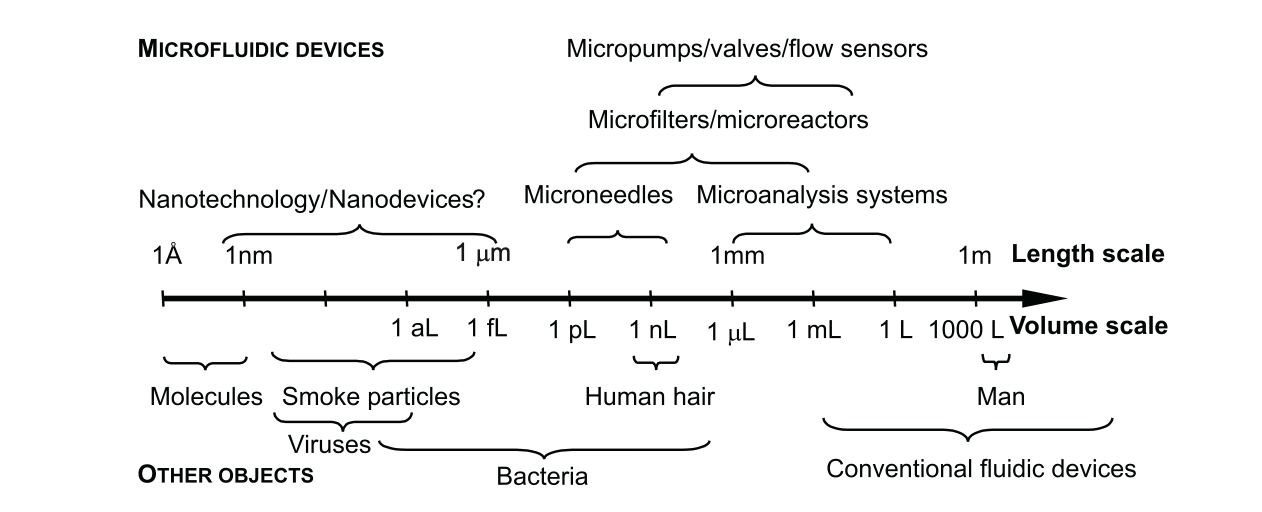
Microfluidic device sizes vary greatly depending on their applications (Photo: Nguyen et al., 2019)
Factors affecting the commercialization of Microfluidic devices
While Microfluidic applications offer the potential to shorten turnaround times and lower costs for analytical instruments, particularly in the fields of medicine, veterinary medicine, and environmental sciences, this breakthrough technology has only just made its way into consumer goods. Several businesses have sprung up to commercialise microfluidic technology during the past 20 years, with the initial intention for biological studies and chemical synthesis, such that a variety of substances may be created and analysed in small amounts to replace manual processing and large benchtop apparatus. Since then, various innovative businesses have appreciated the lower costs of microfluidic devices when compared to traditional bench-top equipment due to the efficient consumption of reagents, high-throughput studies, miniaturisation of components, and ability to create microfluidic devices from inexpensive materials (Blow, Microfluidics: The Great Divide). Some of the reasons for this slow commercialization growth is the lack of additional training, external equipment (pumps, pneumatic fluid handling systems, etc.), hence hindering the mass applications of microfluidics outside the laboratory, especially in genomics and Point-of-Care (POC) diagnostics.
Many successful microfluidic businesses have been able to combine the product development and customer development methodologies simultaneously by concentrating primarily on market entrance strategies. Despite being a viable laboratory tool, microfluidics is still looking for the greatest applications, and with the lack of microfluidics devices with revenues of more than $100 million, many investors lean towards investing in other cutting-edge technologies with established market niches (Blow, Microfluidics: In search of a killer application). The standardisation and integration of microfluidic devices are two areas that need to receive more attention in order to overcome the challenges to commercialization.
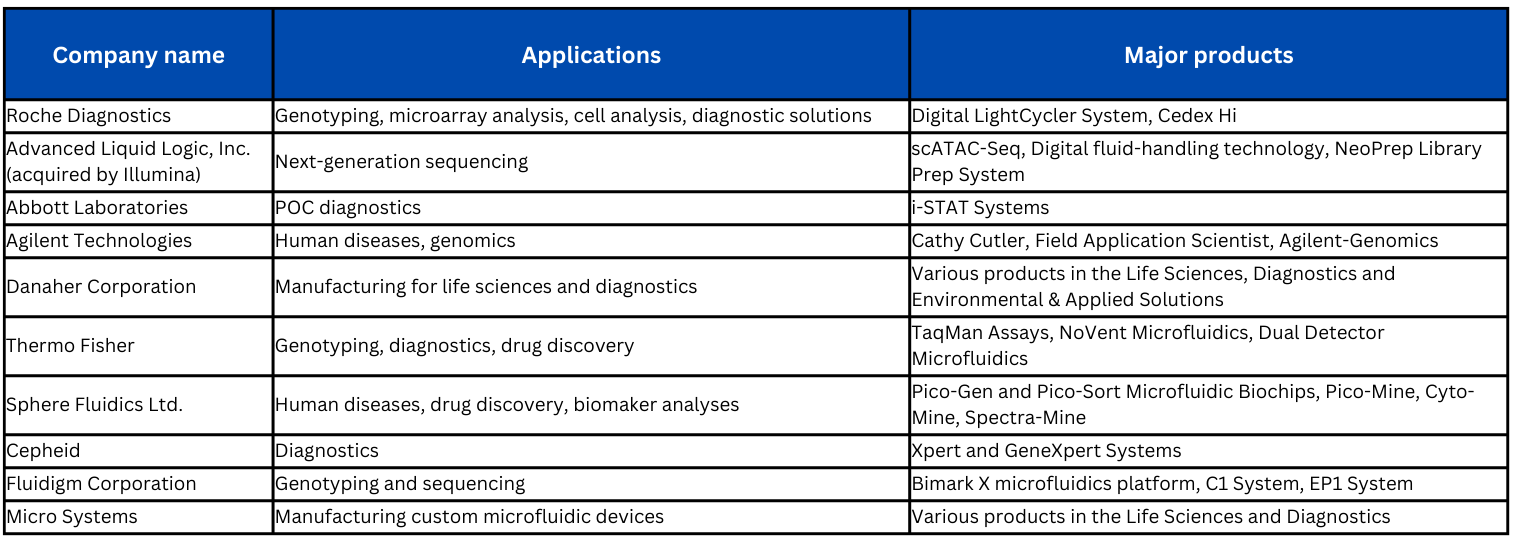
Some leading companies in the innovation and manufacturing of microfluidic devices
In addition, there has been a lack of cooperation between the development and integration of microfluidics components, at the same time, the academic researches are random and unfocused. The success of microfluidics commercialization requires researchers and engineers to work together from the very first step of the design stage, such that the final design for end-users could exploit the full potential of LOC devices at a reasonable, affordable cost. In order to promote the spread of microfluidics as a technology, industry maturation and academic endeavours should be in more corporation. Industrial partners with marketing know-how should participate actively in discussions about a product’s potential for success in a particular market and offer comments on end-user needs and expectations, in contrast, academic researchers can concentrate on producing a fully integrated product with a particular application. Finding a balance between academic publishing, engaging with business partners, and obtaining patent protection in order to improve societal effect would also be necessary in such an endeavour.
Furthermore, in the last decade, hundreds of articles have been published on microfluidics, and the number of publications per year is rising, according to (Mark et al., 2010), but only a small number of practical microfluidic devices are having an influence on society (Van den Berg et al., 2010). Users are clearly more interested in how technology translates to function in their lives, therefore, academia must overcome the overemphasis on science and publishing cutting-edge technology, and instead, focus on creating practical and commercial applications that benefit the larger world.
Another key factor is standardization for microfluidic-based medical devices, which offer the guidelines for dimension and performance control between microfluidic connections, accurate flow rate monitoring, trustworthy testing of various parts or device characteristics, and a modular method to integrating microfluidic, electric, and optical functions (Reyes et al., 2021). So far, there have been some standards developed by ISO (International Organisation for Standardisation), greatly benefiting in creating a controlled and unified supply chain in business. From a production perspective, it eliminates the unnecessary labour and time in the device design process, particularly prototypes. Nevertheless, setting up a standardization system is complicated, especially in the field of microfluidics when there are so many possible uses and manufacturing methods, hence more joint efforts between researchers, designers and engineers are required.
Other factors hindering the commercialization of microfluidic devices include long development period (Dekker et al., 2018), high failure rate and complicated production method (Tian) and high cost of R&D and manufacturing (Nguyen et al., 2018).
For the commercialization of microfluidic devices in the coming years, microfluidic researchers and engineers are aiming for inventions at a lower cost with a higher application level on a larger scale, based on the combined emerging technologies to address real-world issues.
References:
Nguyen, N.-T., Wereley, S.T. and Mousavi, S.S.A. (2019) Fundamentals and applications of Microfluidics. Boston: Artech House.
Blow, N. (no date) Microfluidics: The Great Divide, Nature News. Available at: https://www.nature.com/articles/nmeth0909-683 (Accessed: 30 June 2023).
Blow, N. (no date a) Microfluidics: In search of a killer application, Nature News. Available at: https://www.nature.com/articles/nmeth0807-665 (Accessed: 30 June 2023).
MARK, D., HAEBERLE, S., ROTH, G., VON STETTEN, F., & ZENGERLE, R. (2010). Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chem Soc Rev, 39(3), pp.1153-1182.
VAN DEN BERG, A., CRAIGHEAD, H. G., & YANG, P. (2010). From microfluidic applications to nanofluidic phenomena. Chemical Society Reviews, 39(3), pp.899-900.
Reyes D. R. et al., “Accelerating innovation and commercialization through standardization of microfluidic-based medical devices,” Lab Chip 21, 9–21 (2021). 10.1039/D0LC00963F
Dekker S. et al., “Standardized and modular microfluidic platform for fast lab on chip system development,” Sens. Actuators B 272, 468–478 (2018). 10.1016/j.snb.2018.04.005
Niculescu A. G., Chircov C., Bîrcă A. C., and Grumezescu A. M., “Fabrication and applications of microfluidic devices: A review,” Int. J. Mol. Sci. 22, 2011 (2021). 10.3390/ijms22042011
Nguyen H. T., Thach H., Roy E., Huynh K., and Perrault C. M. T., “Low-cost, accessible fabrication methods for microfluidics research in low-resource settings,” Micromachines 9, 461 (2018). 10.3390/mi9090461
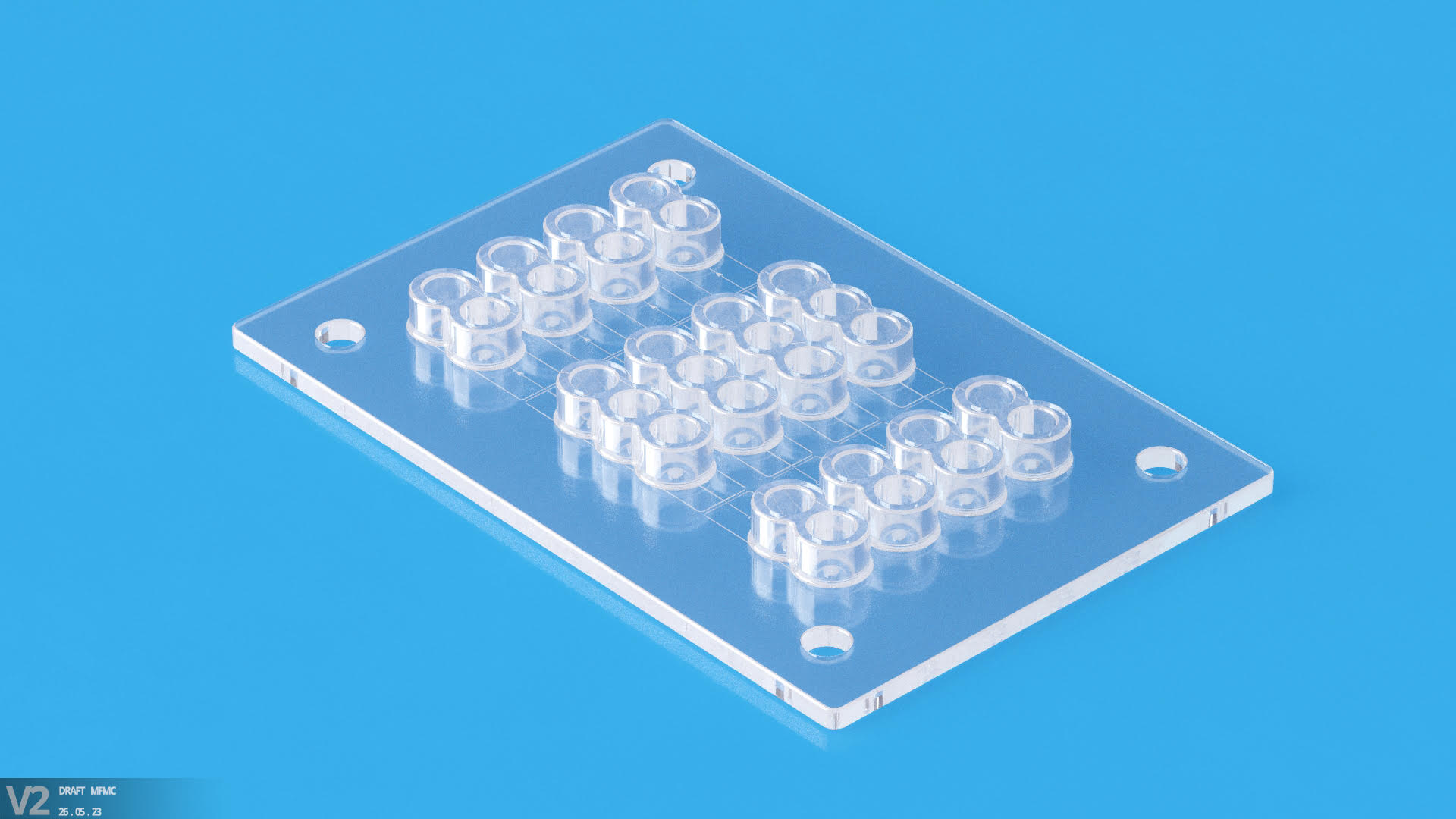
Micro Systems’s vast know-how in design, ultra-precision micro machining capabilities and expert knowledge in micro molding technology allow us to manufacture advanced microfluidic molds with tolerance as low as +/-0.001mm, with integrated optics. We have a dedicated micro molding facility, and have ISO13485 and ISO9001 certifications. For more information, please Contact us or visit our website.