Using Moldflow Analysis to Validate Medical Device Designs Before Tooling
In the highly regulated medical device manufacturing sector, design validation is critical. Before investing in expensive injection mold tooling, many engineering teams now use Moldflow analysis to identify potential manufacturing issues, optimize part geometry, and ensure compliance with UK and EU regulatory standards (Bhattacharjee, 2020; Autodesk, 2023). This simulation-driven approach can significantly reduce risk, shorten lead times, and deliver higher-quality medical devices.
What is Moldflow Analysis in Medical Device Design?
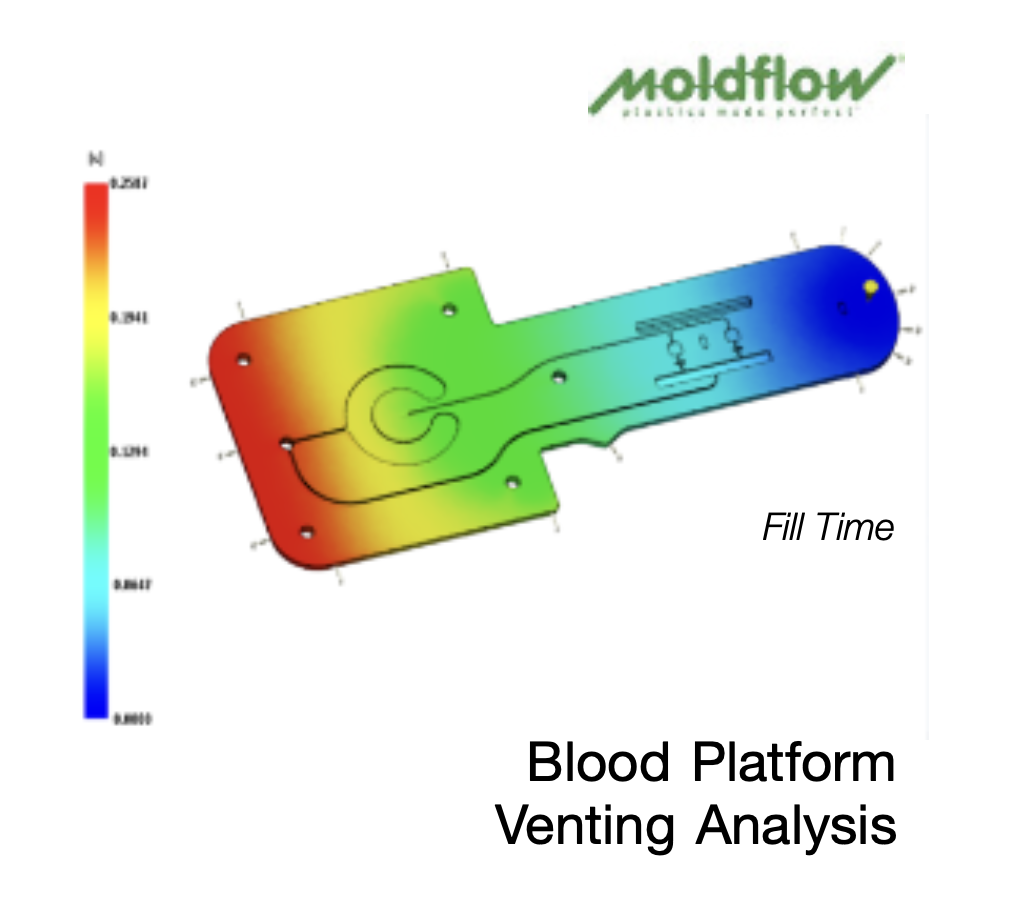
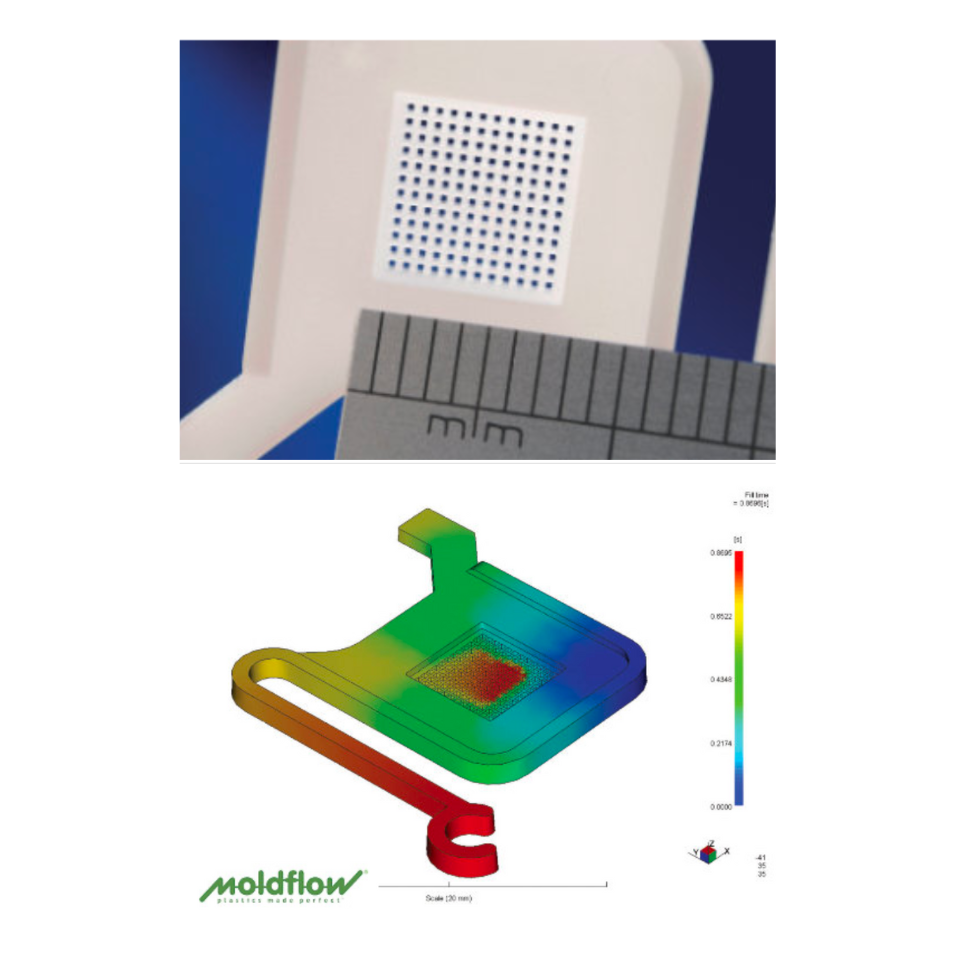
Moldflow analysis is an advanced computer-aided engineering (CAE) tool that predicts how molten plastic flows, cools, and solidifies during the injection molding process. Using 3D CAD data, it generates accurate virtual models that help engineers evaluate:
-
Filling patterns: spotting areas prone to short shots or hesitation.
-
Air traps and weld lines: identifying weaknesses that may compromise performance.
-
Sink marks and warpage: forecasting post-cooling deformation.
-
Cooling efficiency: determining cycle time and thermal distribution.
-
Material behavior: predicting shrinkage rates and processing tolerances.
By detecting these issues before physical tooling begins, engineers can avoid costly re-engineering work (Rosato & Rosato, 2021).
Why Moldflow Analysis is Essential for Medical Devices
Medical devices often feature thin-wall designs, intricate geometries, and demanding tolerances. They must comply with ISO 13485, the UK Medical Devices Regulations, and the EU MDR, meaning manufacturing precision is paramount. In this context, Moldflow analysis offers three major benefits:
-
Risk Mitigation: Predicting defects in advance helps avoid non-compliance and production delays (Gibson et al., 2015).
-
Cost Efficiency: Tooling changes can be expensive; simulation allows modifications while the part exists only as a CAD model (Autodesk, 2023).
-
Faster Market Launch: Regulatory submissions progress more smoothly when designs are validated early (Bhattacharjee, 2020).
Integrating Moldflow Analysis into the Design Workflow
For maximum benefit, Moldflow analysis should be introduced before design freeze. A typical process includes:
-
CAD Model Preparation: Importing the part and mold geometry into the Moldflow software.
-
Material Selection: Choosing compliant medical-grade polymers with proven biocompatibility.
-
Process Definition: Setting gate locations, injection pressures, cooling layouts, and cycle times.
-
Simulation Testing: Running multiple scenarios to assess geometry and process variations.
-
Design Refinement: Adjusting geometry, gating, or parameters for optimal manufacturability.
-
Final Validation: Conducting a final simulation before investing in tooling.
Best Practices for Moldflow Analysis in Medical Device Manufacturing
-
Collaborate with Toolmakers Early: Share simulation data to ensure aligned cooling strategies and gate placement.
-
Verify Predictions with Physical Trials: Compare first-off molded parts to simulation outputs (Gibson et al., 2015).
-
Maintain Comprehensive Documentation: Regulatory audits require evidence of design verification.
-
Account for Sterilisation: Ensure simulated materials withstand autoclaving, gamma irradiation, or other sterilization methods (ISO, 2021).
Why Micro Systems is the Perfect Partner for Mould Design and Validation
For medical device manufacturers, selecting the right engineering partner is as important as the design process itself. Micro Systems combines deep expertise in precision mold design with advanced Moldflow simulation capabilities, offering a complete solution from initial concept to validated production tooling. With in-house medical-grade manufacturing facilities, ISO 13485 certification, and proven experience in producing components for some of the world’s leading medical brands, Micro Systems ensures:
-
Optimized Design for Manufacturability: Minimizing defects before tooling investment.
-
Faster Regulatory Compliance: Supporting documentation and validation for UK, EU, and FDA submissions.
-
Integrated Prototyping and Testing: Verifying simulation results with real-world performance data.
By partnering with Micro Systems, manufacturers gain not only technical excellence but also a strategic advantage in delivering high-quality medical devices to market faster and with reduced risk.
Using Moldflow analysis to validate medical device designs before tooling is now a best practice, not just a cost-saving exercise. By integrating simulation early, UK and EU medical device manufacturers can reduce production risks, improve quality, and accelerate time-to-market while meeting strict regulatory requirements. In an industry where precision and reliability save lives, Moldflow, combined with the expertise of partners like Micro Systems, provides a clear competitive edge.
References
-
Autodesk. (2023). Moldflow Overview. Autodesk Inc.
-
Bhattacharjee, S. (2020). Design for Manufacturability in Injection Moulding. Springer.
-
Gibson, I., Rosen, D., & Stucker, B. (2015). Additive Manufacturing Technologies. Springer.
-
ISO. (2021). ISO 13485: Medical Devices – Quality Management Systems – Requirements for Regulatory Purposes. International Organization for Standardization.
-
Rosato, D. V., & Rosato, D. V. (2021). Injection Molding Handbook. Springer.